
Every year, millions of patients struggle with swallowing conventional tablets or capsules, leading to compromised adherence and suboptimal therapeutic outcomes. For pediatric, geriatric, and dysphagic populations, even a simple dose can become a challenge. Thinoral® technology steps offer a patient-centric solution that combines rapid disintegration, precise dosing, and ease of use.
The rising demand for patient-friendly dosage forms is not anecdotal. Approximately 14% of community-dwelling older adults experience difficulty swallowing oral medications, a condition known as dysphagia. (Source: PubMed) In pediatric populations, poor palatability remains a significant barrier to medication adherence, with nearly 28% of caregivers reporting that their children frequently refuse medications due to unpleasant taste. (Source: verixiv.org)
Thinoral® technology addresses these challenges by integrating formulation innovation, manufacturing precision, and rigorous quality control into a seamless design-to-delivery process.
As a result of this alignment to patient-centric delivery, oral thin film formulations are expected to have substantial growth in this decade, reaching USD 9 billion by the end of 2035.
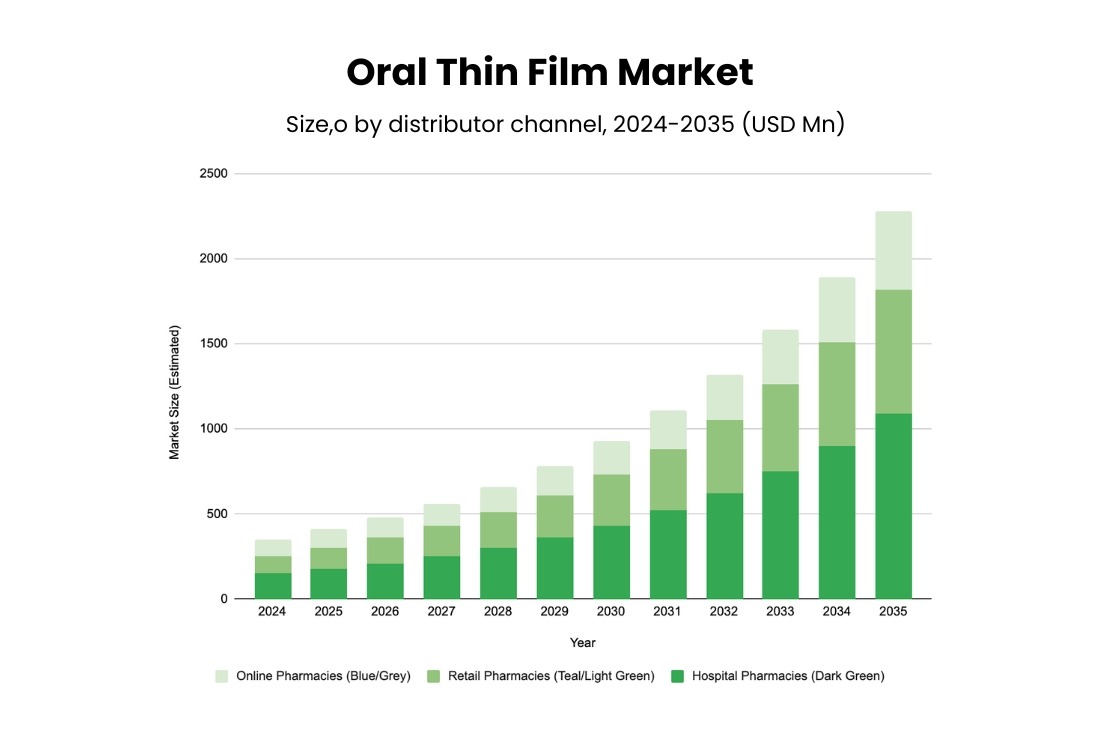
(Source: Transparency Market Research)
Traditional solid oral dosage forms, while reliable, present limitations for certain patient populations. Tablets and capsules often require water, have a slower onset of action, and can discourage adherence when swallowing is difficult. Thinoral® technology overcomes these barriers through fast-dissolving, easy-to-administer films that are suitable for systemic, local, and diagnostic applications.
Key advantages over conventional tablets:
This versatility expands access to medication for populations previously limited by form factor, while supporting global pharmaceutical brands in delivering differentiated, patient-friendly therapies.
To gain a deeper understanding of the absorption advantages, refer to our previous post: How Thinoral® Technology Improves Medication Absorption with Orodispersible Films.
Developing a robust Thinoral product begins at the molecular level. Oral thin film formulation manufacturers must consider several critical factors to ensure optimal performance and patient acceptance.
Key formulation considerations:
Balancing mechanical strength, flexibility, and palatability requires careful optimisation. A film that is too brittle, bendy, or sticky may tear during handling, whereas excessive plasticiser can slow disintegration. Iterative testing during formulation ensures the final product delivers a consistent dose, an acceptable mouthfeel, and ease of administration.
Thinoral® technology enables the production of thin films through advanced manufacturing methods, each with its advantages:
Process parameters, including drying temperature, film thickness, and residual solvent limits, are critical for product quality. Scale-up from lab to commercial production demands a rigorous understanding of material behaviour, equipment capabilities, and process reproducibility.
Oral thin films, like any other pharmaceutical product, undergo a comprehensive quality control program. Key metrics include:
When B2B pharmaceutical manufacturers are involved, robust quality control systems not only guarantee regulatory compliance but also uphold the trust of pharmaceutical companies, which rely on consistent performance across multiple batches and geographies.
The final step in Thinoral® technology is ensuring that the product reaches patients intact and effectively. Moisture-protective packaging is non-negotiable, as even minimal water exposure can compromise disintegration and API stability. Common formats include:
Packaging forms an integral part of the delivery ecosystem, ensuring that the benefits of Thinoral® technology, such as rapid dissolution, precise dosing, and convenience, are preserved until administration.
This comparative view highlights why global pharmaceutical partners are increasingly exploring Thinoral® technology as a strategic addition to their product portfolios.
Thinoral® technology exemplifies the intersection of material science, process innovation, and regulatory rigour in modern pharmaceutical manufacturing. By prioritising patient-centric design while maintaining strict quality control, contract manufacturers can help global brands deliver therapies that are both effective and accessible.
For pharmaceutical companies looking to improve patient adherence, expand therapeutic options, and differentiate in the competitive pharmaceutical market, partnering with a B2B pharmaceutical manufacturer for Thinoral® technology expertise is a compelling, science-driven solution.
ZIM Laboratories Limited is a therapy-agnostic and innovative drug delivery solution provider focusing on enhancing patient convenience and treatment adherence to drug intake. We offer a range of technology-based drug delivery solutions and non-infringing proprietary manufacturing processes to develop, manufacture, and supply innovative and differentiated generic pharmaceutical products to our customers globally. At ZIM Labs, we provide our customers with a comprehensive range of oral solid value-added, differentiated generic products in semi-finished and finished formulations. These include granules, pellets (sustained, modified, and extended-release), taste-masked powders, suspensions, tablets, capsules, and Oral Thin Films (OTF).